Crohn's Disease
Crohn's Disease and How Mesenchymal Stem Cells Can Help
Crohn’s disease is a chronic inflammatory condition that affects the gastrointestinal tract, causing symptoms such as abdominal pain, diarrhea, and weight loss. Despite current treatments such as steroids and biologics, many patients with Crohn’s disease continue to experience symptoms and may eventually require surgery. However, mesenchymal stem cells (MSCs) have shown great promise as a potential treatment for Crohn’s disease, offering a way to reduce inflammation and promote healing in the gut.

Crohn’s disease is believed to be caused by a combination of genetic, environmental, and immune factors. While the exact cause is unknown, it is thought that the immune system mistakenly attacks the cells lining the digestive tract, leading to inflammation and tissue damage. This inflammation can occur anywhere in the digestive tract, from the mouth to the anus, but most commonly affects the small intestine and colon.
The current treatment options for Crohn’s disease include steroids, biologics, and surgery. While steroids can help to control inflammation during a flare-up, they are not a long-term solution due to the risk of side effects such as weakened bones and connective tissue. Biologics, which are chemotherapy-type drugs, can help to prevent flares and reduce inflammation, but they also come with long-term health risks such as an increased risk of cancer. Surgery may be necessary in severe cases to remove damaged tissue or relieve symptoms such as bowel obstruction, but it is not a cure and may still require the use of medications to control inflammation.
Mesenchymal stem cells (MSCs) are a type of stem cell that can be obtained from various sources such as bone marrow, adipose tissue, and umbilical cord blood. MSCs have been found to have immunomodulatory properties, meaning they can help to regulate the immune system and reduce inflammation. This makes them an attractive option for treating Crohn’s disease, as the inflammation in the gut is a key driver of the disease’s symptoms.
MSCs can also differentiate into different types of cells, including those found in the gut. This means that they can help to repair and regenerate damaged tissue in the gastrointestinal tract, further promoting healing and reducing inflammation.
Multiple studies have shown that MSC therapy is an effective and safe treatment option for complex perianal fistulas in patients with Crohn’s disease who have not responded to traditional or biological therapies. In addition, research has shown that MSCs can suppress the pro-inflammatory response in the gut and promote the production of anti-inflammatory cytokines. One study, published in the Journal of Crohn’s and Colitis, found that MSCs reduced the inflammatory response in a mouse model of Crohn’s disease and improved the healing of the gut tissue.
Overall, mesenchymal stem cell therapy shows great promise as a potential treatment option for patients with Crohn’s disease. By reducing inflammation and promoting healing in the gut, MSCs can offer a way to control symptoms and potentially avoid the need for surgery or long-term medication use. While more research is needed to fully understand the long-term efficacy and safety of this treatment, it is an exciting development in the field of gastroenterology and offers hope to those living with this challenging condition.
References
- (1) Rendi, M., & Swanson, P. (2019, November 9). Crohn’s Disease Pathology. Retrieved from https://emedicine.medscape.com/article/1986158-overview#a3
- (2) Mao, Fei, et al. “Mesenchymal Stem Cells and Their Therapeutic Applications in Inflammatory Bowel Disease.” Oncotarget, Impact Journals LLC, 6 June 2017, https://www.ncbi.nlm.nih.gov/pubmed/28402942
- (3) Watt, Fiona M, and Ryan R Driskell. “The Therapeutic Potential of Stem Cells.” Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, The Royal Society, 12 Jan. 2010, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2842697/
- (4) Levy, Michael L., et al. “Phase I/II Study of Safety and Preliminary Efficacy of Intravenous Allogeneic Mesenchymal Stem Cells in Chronic Stroke.” Stroke, vol. 50, no. 10, 2019, pp. 2835–2841., DOI:10.1161/strokeaha.119.026318.
- (5) Zhang XM, Zhang YJ, Wang W, Wei YQ, Deng HX. Mesenchymal Stem Cells to Treat Crohn’s Disease with Fistula. Hum Gene Ther. 2017 Jul;28(7):534-540. doi: 10.1089/hum.2016.095. PMID: 28132518.
- (6) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Crohn’s Disease. (https://www.niddk.nih.gov/health-information/digestive-diseases/crohns-disease) Accessed 5/25/2020.
- (7) Rubin DT, Feld LD, Goeppinger SR, Margolese J, Rosh J, Rubin M, Kim S, Rodriquez DM, Wingate L. The Crohn’s and Colitis Foundation of America Survey of Inflammatory Bowel Disease Patient Health Care Access. Inflamm Bowel Dis. 2017 Feb;23(2):224-232. doi: 10.1097/MIB.0000000000000994. PMID: 27997434.
Diabetes type 1 and 2
There are several types of diabetes. What they all have in common is a problem with regulating normal levels of sugar in the blood. The most common types of diabetes:
Type 1 diabetes occurs when the body’s immune system damages and then destroys beta cells. This means the levels of sugar in the blood stay high all the time, which can lead to long-term damage to the body.
Type 2 diabetes occurs when not enough insulin is made by beta cells or the insulin produced doesn’t work properly (the body’s cells become insulin resistant).
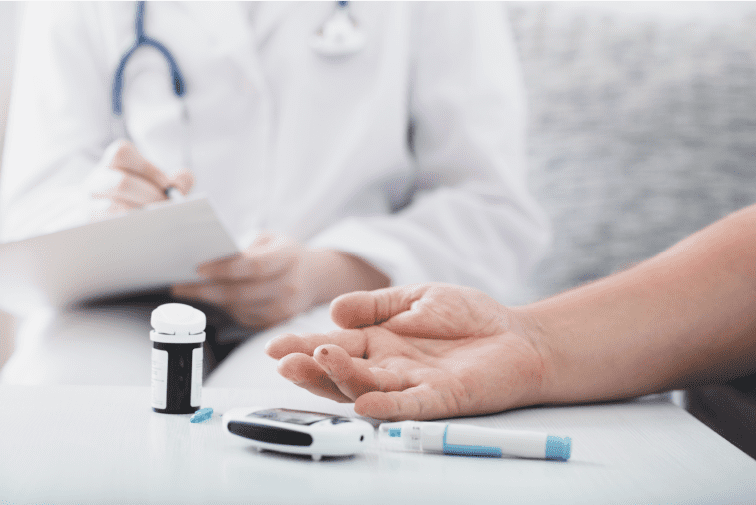
All the cells in your body need energy. This energy is carried around the body as sugar (glucose) in the blood. Normally, blood sugar levels are controlled by the release of the hormone insulin. Insulin is made by cells in the pancreas called beta cells that are arranged into clusters together with other pancreas cells. These clusters are called islets of Langerhans. In one human pancreas there are roughly one million islets.
- Also known as juvenile diabetes
- Stem cells become any type of cell
- Our challenge
By the time a patient is diagnosed with T1D, also known as juvenile diabetes, the destruction of insulin-producing beta cells by the immune system is nearly complete. Because of this, there is no way to discover what it was that led the person’s immune system to attack the beta cells in the first place. Even if it were possible to identify future T1D patients before the immune attack on beta cells began, disease onset and progression could not be studied in these individuals due to the inaccessibility of the pancreas – where beta cells are found – in a living person.
Umbilical cord-derived mesenchymal stem cells (UCMSCs), hold tremendous potential as a source of insulin-producing cells that could be placed into the pancreas. That’s because stem cells have the potential to become virtually any kind of cell.
Our challenge is to “push” stem cells down the path we want them to go – to emerge as cells that sense glucose and secrete insulin. To accomplish that, International Stem Cell Institute® scientists are working with UCMSCs. UCMSCs are able to support beta cell regeneration in damaged pancreas tissue, and as stem cells transplantation procedures have been taking place since the 1960s, Umbilical cord-derived mesenchymal stem cells are a leading candidate for a possible therapy.
CMSCs, a newer stem cell on the block, live in the umbilical cord and other tissues and also have the regenerative potential to repair beta cells. These cells have an intrinsic ability to modulate the immune system. This is important because newly formed beta cells, regardless of how they are made, will need to be protected if they are to avoid the same autoimmune response that attacked the patient’s beta cells in the first place. Currently there are many clinical trials underway to test if hematopoietic stem cells and mesenchymal stem cells can offset the amount of insulin needed by diabetics to manage their blood sugar levels.
Contrary to other Stem Cell treatments based on autologous transplant, where the stem cells are as old as the patient’s age and carry all the information accumulated through their life, at the International Stem Cell Institute®, we use donor umbilical cord-derived mesenchymal stem cells (MSCs) instead of bone marrow or fat tissue-derived. In our laboratory, umbilical cord MSCs can undergo approximately twice the number of “doublings” as their adult-derived bone marrow or fat tissue counterparts. The overall hypothesis is that UCMS cells possess immune properties that would be permissive to allogeneic transplantation. For example, UCMS cells will suppress the proliferation of “stimulated” lymphocytes (immune suppression) and have reduced immunogenicity (e.g., would be poor stimulators of allogeneic lymphocyte proliferation).
Mesenchymal stem cells are progenitors for several connective tissue cell lineages, including bone, cartilage, muscle, fat, and bone marrow. As mesenchymal stem cells can target diseased organs, they may hold potential as vehicles capable of expressing and secreting proteins with therapeutic effects. These conditions are ailments and disorders in muscles, ligaments, and joints. Common examples include Rheumatoid Arthritis, degenerative disc disease, osteoarthritis, and various injuries like fractures and torn cartilage.
International Stem Cell Institute® Stem Cell Procedures
Help overcome these conditions by extracting stem cells from the umbilical cord, then concentrating and culture the cells and injecting them into the damaged area to help the body heal naturally. Our Stem Cell Procedures can be used for a wide range of conditions and are the tool of choice for injuries, arthritis, and other conditions that may be more significant than those treated with conventional therapies. According to our research, stem cells have self-renewal qualities which aid in reproducing cartilage and bone tissue cells, restoring joint function, and reducing pain. Scientists have discovered that stem cells and specifically allogeneic umbilical cord mesenchymal stem cells, are home to inflamed tissue and start producing anti-inflammatory agents. Usually, these cells are harvested from human umbilical cords donated after normal healthy births. This means that stem cell transplants could be used to treat some of the most common osteoarticular symptoms.
- Everything about your International Stem Cell Institute® experience is designed for results.
- At the International Stem Cell Institute®, our priority is to produce the best possible outcomes for our patients.
- And while our protocol is far more complex than you will find at other regenerative medicine clinics, we do not cut corners.
The injection typically takes place three weeks after the UCMSCs are extracted from the umbilical cord. During this time, cells are grown for approximately 15 days and then tested for quality assurance, including sterility testing and karyotype analysis to ensure no genetic abnormalities present in the stem cells. Once the UCMSCs have passed all safety and quality testing, you will be scheduled for the injection procedure. During the injection, the cultured cells are thoroughly tested for quality assurance and injected using guided imaging to ensure the most precise placement of cells into the injured area.
Transform your health with world-renowned
stem cell specialists - Free consultation
Irritable Bowel Syndrome
Irritable Bowel Syndrome (IBS) is a common disorder affecting the large intestine, marked by symptoms like cramping, abdominal pain, bloating, gas, diarrhea, and constipation. It’s a chronic condition that necessitates long-term management. Statistics reveal that IBS affects between 25 and 45 million people in the United States alone, with a significant impact on quality of life and healthcare costs.
Revolutionizing IBS Treatment with Mesenchymal Stem Cells from Wharton’s Jelly (MSCWJ)
At The International Stem Cell Institute, led by Dr. Leonardo Gonzalez, we’re pioneering a transformative approach to treating IBS using Mesenchymal Stem Cells derived from Wharton’s Jelly (MSCWJ). These cells have shown promising potential in regenerative medicine, particularly in treating conditions like IBS.
How MSCWJ Works for IBS
- Regenerative Potentia
- Anti-Inflammatory Properties
- Immunomodulatory Effects
MSCWJ can differentiate into various cell types, aiding in the regeneration and repair of damaged intestinal tissues.
These cells can modulate immune responses, reducing inflammation that is often a key component in IBS.
MSCWJ can potentially recalibrate the immune system, addressing autoimmunity aspects that may contribute to IBS
Dr. Gonzalez’s Unique Approach
In Bogotá, Colombia, due to restrictive regulations in the USA, Dr. Gonzalez offers a unique treatment protocol for IBS:
· Precision Delivery: Using catheterization in a cath lab, Dr. Gonzalez ensures that MSCWJ are delivered precisely to the affected areas of the intestine.
· Customized Treatment: Each patient undergoes a thorough evaluation to tailor the treatment to their specific needs.
Statistics and Impact
· Prevalence of IBS: In America, IBS disproportionately affects women and usually develops before the age of 50.
· Economic Burden: IBS is responsible for significant healthcare expenses and lost productivity, with an estimated direct and indirect cost running into billions annually.
Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a long-lasting autoimmune disorder that primarily affects joints. It typically results in warm, swollen, and painful joints. Pain and stiffness often worsen following rest. Most commonly, the wrist and hands are involved, with the same joints typically involved on both sides of the body. While the cause of rheumatoid arthritis is not clear, it is believed to involve a combination of genetic and environmental factors. The underlying mechanism involves the body’s immune system attacking the joints. This results in inflammation and thickening of the joint capsule. It also affects the underlying bone and cartilage.
Some people have mild or moderate forms of the disease with times when the symptoms get worse and times when they get better. Others have a severe form of the disease that can last for many years or a lifetime. This form of the disease can cause serious joint damage. The goals of RA treatment are stop inflammation (put disease in remission), relieve symptoms, prevent joint and organ damage, improve physical function and overall well-being, and reduce long-term complications.
- Development of therapies
- Stem cells to the areas patients need them most
The advancements in our understanding of the inflammatory and immune mechanisms in rheumatoid arthritis (RA) have fuelled the development of targeted therapies that block cytokine networks and pathogenic immune cells, leading to a considerable improvement in the management of RA patients.
The recent evidence that mesenchymal stem cells (MSCs) with the ability to differentiate into cartilage are present in joint tissues raises an opportunity for therapeutic interventions via targeting intrinsic repair mechanisms.
Under physiological conditions, MSCs in the joint are believed to contribute to the maintenance and repair of joint tissues. In RA, however, the repair function of MSCs appears to be repressed by the inflammatory milieu. In addition to being passive targets, MSCs could interact with the immune system and play an active role in the perpetuation of arthritis and progression of joint damage.
At the International Stem Cell Institute® we are studying potential ways to directly target the conditions and complications themselves. These studies consist of multiple ways to deliver the highest amount of activated stem cells to the areas patients need them most. When stem cells are studied through the International Stem Cell Institute®, as potential therapy for Rheumatoid Arthritis, there are multiple ways they can be administered:
- Full body IV – directed into the vein.
- Direct site injections – image guided injection directly into the site that needs repair, i.e., muscles and tendons.
Contrary to other Stem Cell treatments based on autologous transplant, where the stem cells are as old as the patient’s age and carry all the information accumulated through their life, at the International Stem Cell Institute®, we use donor umbilical cord-derived mesenchymal stem cells (MSCs) instead of bone marrow or fat tissue-derived. In our laboratory, umbilical cord MSCs can undergo approximately twice the number of “doublings” as their adult-derived bone marrow or fat tissue counterparts. The overall hypothesis is that UCMS cells possess immune properties that would be permissive to allogeneic transplantation. For example, UCMS cells will suppress the proliferation of “stimulated” lymphocytes (immune suppression) and have reduced immunogenicity (e.g., would be poor stimulators of allogeneic lymphocyte proliferation).
Mesenchymal stem cells are progenitors for several connective tissue cell lineages, including bone, cartilage, muscle, fat, and bone marrow. As mesenchymal stem cells can target diseased organs, they may hold potential as vehicles capable of expressing and secreting proteins with therapeutic effects. These conditions are ailments and disorders in muscles, ligaments, and joints. Common examples include Rheumatoid Arthritis, degenerative disc disease, osteoarthritis, and various injuries like fractures and torn cartilage.
International Stem Cell Institute® Stem Cell Procedures
Help overcome these conditions by extracting stem cells from the umbilical cord, then concentrating and culture the cells and injecting them into the damaged area to help the body heal naturally. Our Stem Cell Procedures can be used for a wide range of conditions and are the tool of choice for injuries, arthritis, and other conditions that may be more significant than those treated with conventional therapies. According to our research, stem cells have self-renewal qualities which aid in reproducing cartilage and bone tissue cells, restoring joint function, and reducing pain. Scientists have discovered that stem cells and specifically allogeneic umbilical cord mesenchymal stem cells, are home to inflamed tissue and start producing anti-inflammatory agents. Usually, these cells are harvested from human umbilical cords donated after normal healthy births. This means that stem cell transplants could be used to treat some of the most common osteoarticular symptoms.
- Everything about your International Stem Cell Institute® experience is designed for results.
- At the International Stem Cell Institute®, our priority is to produce the best possible outcomes for our patients.
- And while our protocol is far more complex than you will find at other regenerative medicine clinics, we do not cut corners.
The injection typically takes place three weeks after the UCMSCs are extracted from the umbilical cord. During this time, cells are grown for approximately 15 days and then tested for quality assurance, including sterility testing and karyotype analysis to ensure no genetic abnormalities present in the stem cells. Once the UCMSCs have passed all safety and quality testing, you will be scheduled for the injection procedure. During the injection, the cultured cells are thoroughly tested for quality assurance and injected using guided imaging to ensure the most precise placement of cells into the injured area.
Join us on a journey to better health, where we go beyond
conventional treatments to provide lasting solutions.
Multiple Sclerosis
Multiple sclerosis (MS) is a demyelinating disease in which the insulating covers of nerve cells in the brain and spinal cord are damaged. This damage disrupts the ability of parts of the nervous system to communicate, resulting in a range of signs and symptoms, including physical, mental, and sometimes psychiatric problems. Specific symptoms can include double vision, blindness in one eye, muscle weakness, trouble with sensation, or trouble with coordination.
MS takes several forms, with new symptoms either occurring in isolated attacks (relapsing forms) or building up over time (progressive forms).

Between attacks, symptoms may disappear completely; however, permanent neurological problems often remain, especially as the disease advances. Most people are diagnosed between the ages of 20-40, but it can affect younger and older people too.
- What current the International Stem Cell Institute research found
- Because our treatment is better
The International Stem Cell Institute®´s current research found that stem cells are able to repair damages of the neural myelin and axons while the mesenchymal stem cells are able to adjust the autoimmune status, which can slow down or even halt the progression of the disease while repairing the neural lesions that already exist. It is important to emphasize that the cellular therapy cannot be the only method used, otherwise the implantation of cells cannot survive or have normal functions. In order to achieve the best outcome, one should combine taking certain amounts of medication and doing physical therapy.
The UCMSCs used by the International Stem Cell Institute® to treat MS have a positive impact on all of the body’s organs and systems, starting with the brain. Our treatment results in better blood and oxygen flow to the brain, called “improved perfusion,” better replacement of damaged neurons, and better formation of new arteries. After a certain period, UCMSCs acquire the properties of the surrounding cells and differentiate into them. This leads to the restoration of white and gray matter and, consequently, to the subsiding of neurological symptoms and to improved brain performance.
Contrary to other Stem Cell treatments based on autologous transplant, where the stem cells are as old as the patient’s age and carry all the information accumulated through their life, at the International Stem Cell Institute®, we use donor umbilical cord-derived mesenchymal stem cells (MSCs) instead of bone marrow or fat tissue-derived. In our laboratory, umbilical cord MSCs can undergo approximately twice the number of “doublings” as their adult-derived bone marrow or fat tissue counterparts. The overall hypothesis is that UCMS cells possess immune properties that would be permissive to allogeneic transplantation. For example, UCMS cells will suppress the proliferation of “stimulated” lymphocytes (immune suppression) and have reduced immunogenicity (e.g., would be poor stimulators of allogeneic lymphocyte proliferation).
Mesenchymal stem cells are progenitors for several connective tissue cell lineages, including bone, cartilage, muscle, fat, and bone marrow. As mesenchymal stem cells can target diseased organs, they may hold potential as vehicles capable of expressing and secreting proteins with therapeutic effects. These conditions are ailments and disorders in muscles, ligaments, and joints. Common examples include Rheumatoid Arthritis, degenerative disc disease, osteoarthritis, and various injuries like fractures and torn cartilage.
International Stem Cell Institute® Stem Cell Procedures
Help overcome these conditions by extracting stem cells from the umbilical cord, then concentrating and culture the cells and injecting them into the damaged area to help the body heal naturally. Our Stem Cell Procedures can be used for a wide range of conditions and are the tool of choice for injuries, arthritis, and other conditions that may be more significant than those treated with conventional therapies. According to our research, stem cells have self-renewal qualities which aid in reproducing cartilage and bone tissue cells, restoring joint function, and reducing pain. Scientists have discovered that stem cells and specifically allogeneic umbilical cord mesenchymal stem cells, are home to inflamed tissue and start producing anti-inflammatory agents. Usually, these cells are harvested from human umbilical cords donated after normal healthy births. This means that stem cell transplants could be used to treat some of the most common osteoarticular symptoms.
- Everything about your International Stem Cell Institute® experience is designed for results.
- At International Stem Cell Institute®, our priority is to produce the best possible outcomes for our patients.
- And while our protocol is far more complex than you will find at other regenerative medicine clinics, we do not cut corners.
The injection typically takes place three weeks after the UCMSCs are extracted from the umbilical cord. During this time, cells are grown for approximately 15 days and then tested for quality assurance, including sterility testing and karyotype analysis to ensure no genetic abnormalities present in the stem cells. Once the UCMSCs have passed all safety and quality testing, you will be scheduled for the injection procedure. During the injection, the cultured cells are thoroughly tested for quality assurance and injected using guided imaging to ensure the most precise placement of cells into the injured area.
Empower yourself with knowledge about cutting-edge treatments for autoimmune diseases through in-depth studies and research. Uncover the transformative potential of stem cell therapies in addressing autoimmune conditions. Your path to informed decision-making starts with understanding the latest research. Click here to explore the studies and embark on a journey towards a more comprehensive approach to managing autoimmune diseases. Take the first step in revolutionizing your health and well-being.
2023 © Mercadeo Salud. All rights reserved.

